
4 hour delivery if ordered by 3pm Click and collect from over 200 stores Genie 58158 Fork Extension 2kg Each Code: 71/5031-h + ADD TO WISHLIST The Genie 58158 fork extensions are designed to increase the length of the material lift standard forks to give a more stable platform for raising/lowering larger/bulkier items.Genie® Forks 48 in, 60 in, 72 in | 122 cm, 152 cm, 183 cm Shaft-Mounted forks for lifting, moving and maneuvering pallets and other loads with ease.

Bring out the tape and extension cords! 3.0k. Grab a fork lift and use the fork access holes to move it. A 19 foot scissor lift is about 8 or 900 lbs.
#Lattice energy of nacl manual#
IIRC it should have a manual release so you can push it. Shaft Size.Looks like a relatively small genie scissor lift.

Choose an option 48 inches 60 inches 72 inches 84 inches 96 inches. Choose an option Genie/Terex 1048 Genie/Terex 5519 Genie/Terex 642 Genie/Terex SS636C Genie/Terex TH1056C Genie/Terex TH644C Genie/Terex TH842C.
#Lattice energy of nacl full#
Available with a full range of useful accessories including extension forks, pipe cradle, boom, barrel stacker and platform.Make/Model. This heavy-duty, manually operated lift is suitable for warehouse work and on-site tasks such as ducting and ventilation installation. Genie Super Lift Advantage Lifter Stacker. Add Genie 80679GT Fork Extension Pin to complete unit. Sold individually, must buy 2 to make complete unit. Shaft Size.Fork Extension: insert onto standard or adjustable forks for additional lengths up to 25 in. Suitable for use with Genie Material Lift Allows the forks to be extended by 700mm These Will Decrease The Load Capacity, refer to user guide for more information Technical specification Brand Name TP Hire Make/Model. Note ** These will decrease load capacity.
#Lattice energy of nacl free#
Ε 0 = permittivity of free space, equal to 8.854×10 −12 C2 J −1 m −1 Īnd n is the Born exponent, where 5

Usually, Ionic Compounds with smaller energy tend to be more soluble in H 2O Born-Lande Equation: Lattice Energy The two main factors that contribute to the energy of an ionic solid is: Usually, this energy for solids such as sugar/iodine whose neutral molecules interact by weaker dipole-dipole or Vander Waal forces is considerably less in magnitude when compared to sodium chloride and such crystals, metals like iron, covalently linked materials like a diamond. As per this convention, the energy of NaCl would be +786 KJ/Mol. Sometimes, in a few textbooks, this energy is defined with the opposite sign (i.e., positive) because of the energy required to convert the crystal into infinitely separated gaseous ions in the vacuum, an endothermic process.
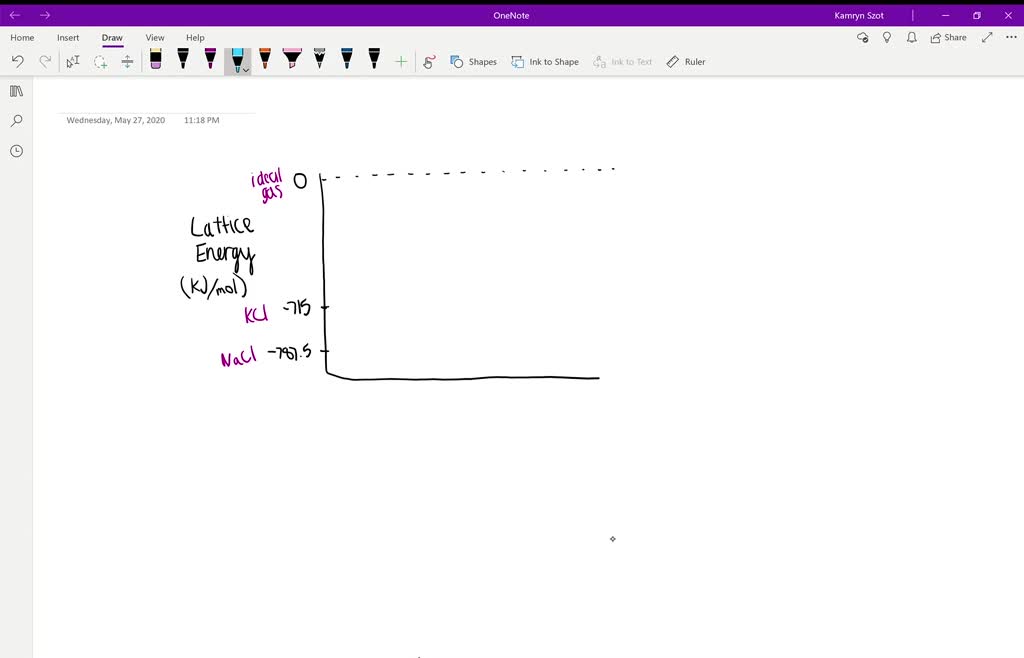
Just like Sodium Chloride, a few compounds energy is listed below: Compound The energy released by this reaction, which is also can be termed as Lattice energy is -786 KJ/mol. The concept of this energy was originally developed for sphalerite and rock salt-structured compounds where the ions occupy high-symmetry crystal lattice sites such as NaCl, ZnS. Often, the energy is exothermic, i.e., the value of ΔH lattice is negative because it corresponds to the coalescing of infinitely separated gaseous ions in vacuum to form the ionic lattice. It is usually deduced from the Born-Haber cycle. It is relevant to many properties such as solubility, hardness, and volatility. We can also describe it as a measure of the cohesive forces that bind ions. Lattice Energy, for a crystalline solid, is the measure of the energy released when ions are combined to make a compound.


 0 kommentar(er)
0 kommentar(er)
